|1,564 Days of Dedication: EchoVIU Officially Receives tFDA Approval🌟🏆
← Back to News List
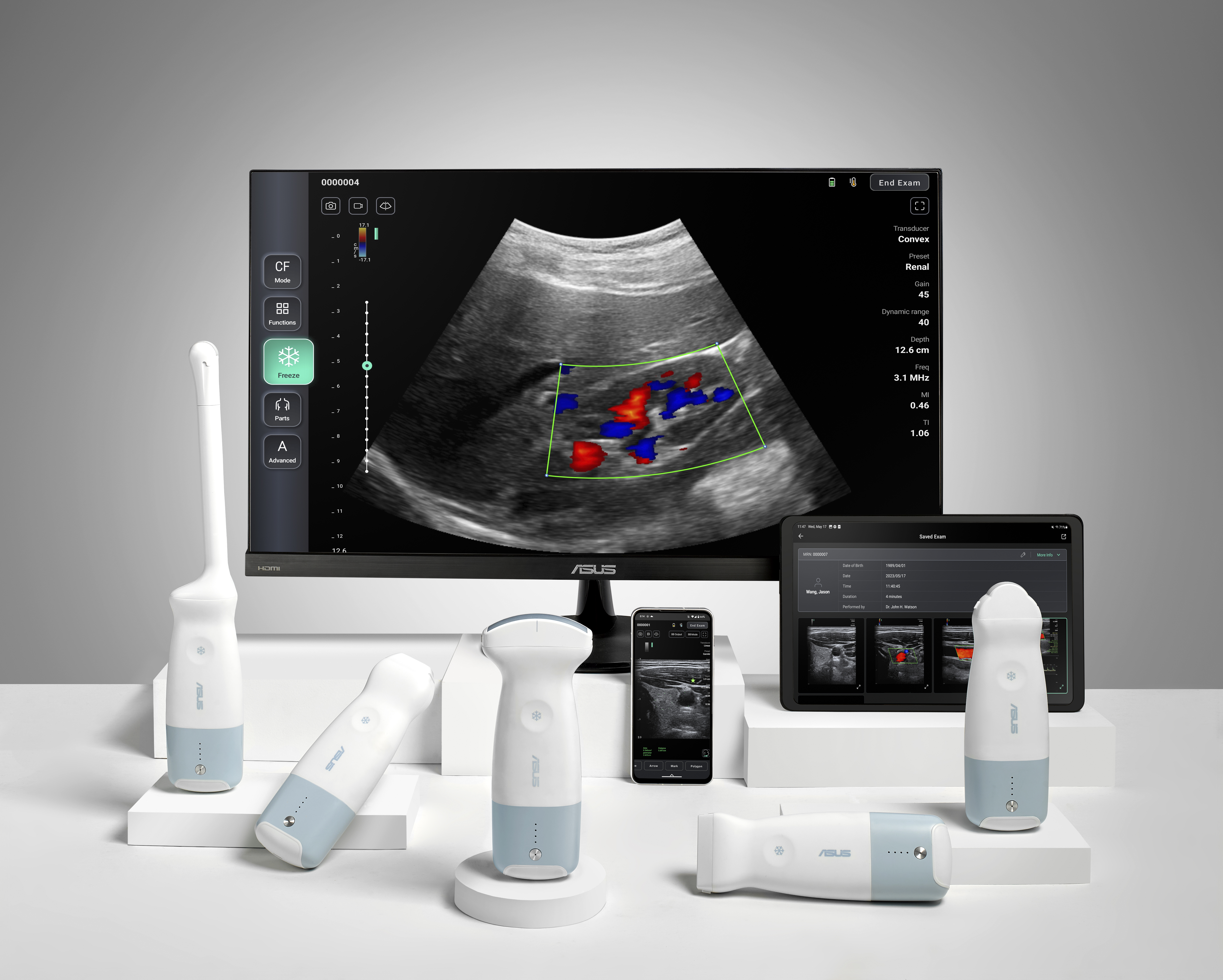
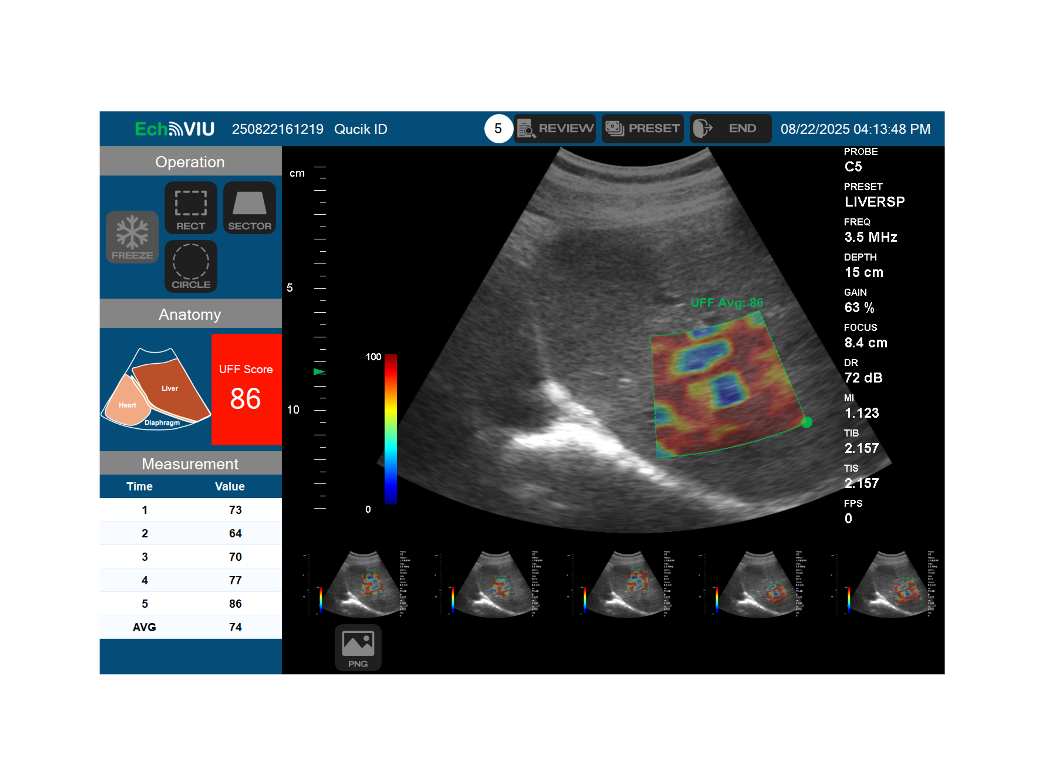
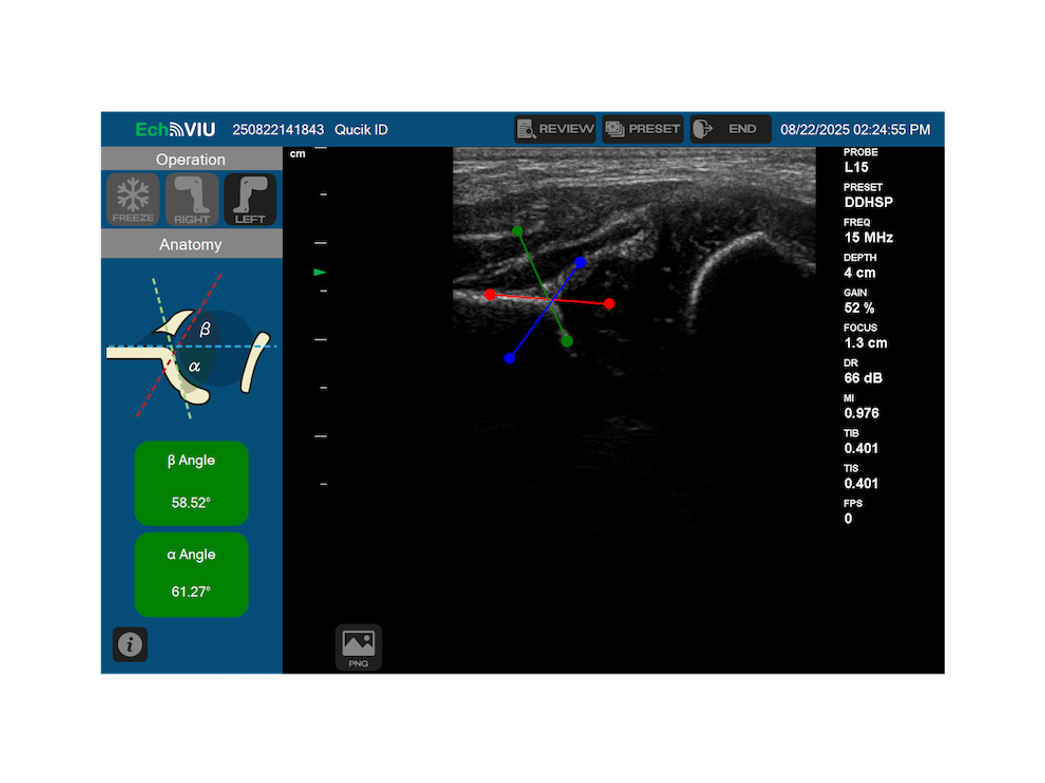
After 1,564 days of development and regulatory effort  , EchoVIU has officially received medical device approval from the Taiwan Food and Drug Administration (tFDA)
, EchoVIU has officially received medical device approval from the Taiwan Food and Drug Administration (tFDA) 

This milestone reflects Echo-Int’s long-term commitment to product quality, regulatory compliance, and clinical value 
Echo-Int will continue advancing clinical-focused innovation  and global collaboration
and global collaboration  , delivering reliable ultrasound solutions for healthcare professionals
, delivering reliable ultrasound solutions for healthcare professionals 
Published on: 2025-12-02